Benjamin Mateus
In the course of a lifetime, the human heart will beat more than three billion times. We will have taken more than 670 million breaths before we reach the end of our lives. Yet, these critical events remain unconscious and imperceptible in everyday life, unless we exert ourselves, such as running up several flights of stairs. We quickly tire, stop to take deep breaths and become flushed.
With the deepening comprehension by medical science of how our bodies work, we have come to better understand the fundamental importance of oxygen to life. Every living organism relies on it in one form or another. However, how cells and tissues can monitor and respond to oxygen levels remained difficult to elucidate. It has only been late in the 20th century with advances in cellular biology and scientific instrumentation that these processes have finally been explained.
On Monday, the 2019 Nobel Prize in Physiology or Medicine was awarded jointly to three individuals: William G. Kaelin, Jr., Sir Peter J. Ratcliffe, and Gregg L. Semenza. Specifically, their discoveries helped elucidate the mechanisms for life’s most basic physiologic processes.
 The official announcement of the laureats. Credit: Nobel Prize Institute
The official announcement of the laureats. Credit: Nobel Prize Institute
They were able to discover how oxygen levels directly affect cellular metabolism, which ultimately controls physiological functions. More importantly, their findings have significant implications for the treatments of conditions as varied as chronic low blood counts, kidney disease, patients with heart attacks or stroke and cancers. One of the hallmarks of cancer is its ability to generate new blood vessels to help sustain its growth. It also uses these oxygen cellular mechanisms to survive in low oxygen environments.
Dr. William G. Kaelin Jr. is a professor of medicine at Harvard University and the Dana-Farber Cancer Institute. The main focus of his work is on studying how mutations in what are called tumor suppressor genes lead to cancer development. Tumor suppressor genes are special segments of the DNA whose function is to check the integrity of the DNA before allowing a copy of itself to be made and undergo cell division, which prevents cells from propagating errors. Cellular mechanisms are then recruited to fix these errors or drive the cell to destroy itself if the damage is too severe or irreparable.
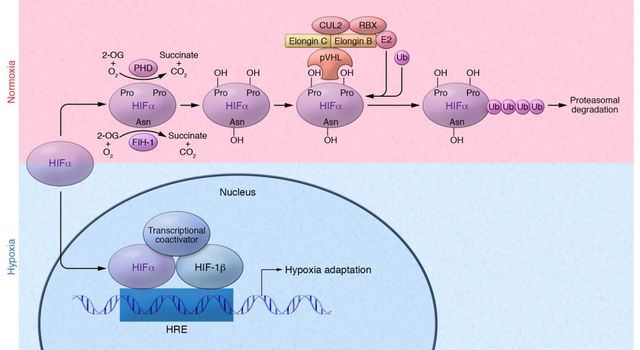
His interest in a rare genetic disorder called Von Hippel-Lindau disease (VHL) led him to discover that cancer cells that lacked the VHL gene expressed abnormally high levels of hypoxia-regulated genes. The protein called the Hypoxia-Inducible Factor (HIF) complex was first discovered in 1995 by Gregg L. Semenza, a co-recipient of the Nobel Prize. This complex is nearly ubiquitous to all oxygen-breathing species.
The function of the HIF complex in a condition of low oxygen concentration is to keep cells from dividing and growing, placing them in a state of rest. However, it also signals the formation of blood vessels, which is important in wound healing as well as promoting the growth of blood vessels in developing embryos. In cancer cells, the HIF complex helps stimulate a process called angiogenesis, the formation of new blood vessels, which allows the cancer cells to access nutrition and process their metabolic waste, aiding in their growth. When the VHL gene is reintroduced back into the cancer cells, the activity of the hypoxia-regulated genes returns to normal.
Dr. Gregg L. Semenza is the founding director of the vascular program at the Johns Hopkins Institute for Cell Engineering. He completed his residency in pediatrics at Duke University Hospital and followed this with a postdoctoral fellowship at Johns Hopkins. His research in biologic adaptations to low oxygen levels led him to study how the production of erythropoietin (EPO) was controlled by oxygen. EPO is a hormone secreted by our kidneys in response to anemia. The secretion of EPO signals our bone marrow to produce more red blood cells.
 A diagram showing how cells make use of oxygen. Credit: Nobel Prize Institute
A diagram showing how cells make use of oxygen. Credit: Nobel Prize Institute
His cellular and mouse model studies identified a specific DNA segment located next to the EPO gene that seemed to mediate the production of EPO under conditions of low oxygen concentration. He called this DNA segment HIF.
Sir Peter J. Ratcliffe, a physician and scientist, trained as a nephrologist, was head of the Nuffield Department of Clinical Medicine at the University of Oxford until 2016, when he became Clinical Research Director at the Francis Crick Institute. Through his research on the cellular mechanisms of EPO and its interaction between the kidneys and red cell production, he found that these mechanisms for cellular detection of hypoxia, a state of low oxygen concentration, were also present in several other organs such as the spleen and brain. Virtually all tissues could sense oxygen in their micro-environment, and they could be modified to give them oxygen-sensing capabilities.
Dr. Kaelin’s findings had shown that the protein made by the VHL gene was somehow involved in controlling the response to low oxygen concentrations. Dr. Ratcliffe and his group made the connection through their discovery that the protein made by the VHL gene physically interacts with HIF complex, marking it for degradation at normal oxygen levels.
In 2001, both groups published similar findings that demonstrated cells under normal oxygen levels will attach a small molecular tag to the HIF complex that allows the VHL protein to recognize and bind HIF, marking it for degradation by enzymes. If the oxygen concentration is low, the HIF complex is protected from destruction. It begins to accumulate in the nucleus where it binds to a specific section of the DNA called hypoxia-regulating genes, which sets into motion the necessary mechanisms to respond to the low oxygen concentration.
The ability to sense oxygen plays a vital role in health and various disease states. Patients who suffer from chronic kidney failure also suffer from severe anemia because their ability to produce EPO is limited. This hormone is necessary for the stem cells in our bone marrow to produce red blood cells. Understanding how cancer cells utilize oxygen-sensing mechanisms has led to a variety of treatments that targets these pathways. The ability to elucidate these mechanisms offers insight into directions scientists and researchers can take to design or create novel treatments.
No comments:
Post a Comment