Benjamin Mateus
New research in the study of the human genome has provided a new way to reduce or potentially eliminate inherited genetic disorders, such as those that lead to a higher risk of diabetes or cancer. This new method, published in the journal Nature, focuses on correcting harmful genetic mutations while the subject is still an embryo.
The findings were spearheaded by an international team led by researchers at the Oregon Health and Science University and included scientists from China, the Republic of Korea and the United States. They focused on a particular disorder known as familial hypertrophic cardiomyopathy (HCM), an enlarged heart, and used a technique known as CRISPR–Cas9 to fix the faulty part of the embryo’s DNA that eventually causes the disorder.
 Illustration of a human embryo at the 8-cell stage
Illustration of a human embryo at the 8-cell stage
HCM leads to sudden death in one in every 500 young athletes, and in 40 percent of cases is caused by a defect in the gene MYBPC3. It is one of the more than 10,000 inherited disorders caused by a single defective gene, which in total affect millions of people worldwide. Besides an enlarged heart, such mutations can develop into breast and ovarian cancer, sickle cell disease, cystic fibrosis, polycystic kidney disease, and Tay-Sachs disease. Moreover, such diseases tend to manifest later in a person’s life, generally after they’ve had children, causing such problems to be unknowingly passed on to the next generation.
Presently, couples who wish to avoid passing such defects to their children and can afford in-vitro fertilization must have their embryos tested, selecting out the ones with the “bad” genes and attempting to conceive with the “healthy” ones.
Using the example of HCM, there is a 50 percent chance of the embryos inheriting the defective gene, Statistically, half the embryos should be mutation free. Correcting the gene could rescue the embryos carrying the defective gene, increase the number of embryos for transfer and improve pregnancy rates. Corrected embryos also imply that future generations would be spared the impact of this mutation on their life as well as those of their offspring.
The present study suggests that CRISPR-Cas9 is an effective and safe way to correct the DNA in these embryos. CRISPR-Cas9 is a molecular tool that can recognize specific genetic sequences and induce a break in the DNA and excise it. The cell then uses internal DNA repair mechanisms to attempt to correct this excised portion. One commonly employed mechanism, non-homologous end-joining (NHEJ) pathways, is prone to introducing additional mutations. There is also an alternative mechanism called homology-directed repair (HDR) that rebuilds the DNA using the non-mutant homologous chromosome (the genes inherited from the other parent) as a template which allows for a correct copy to be inserted.
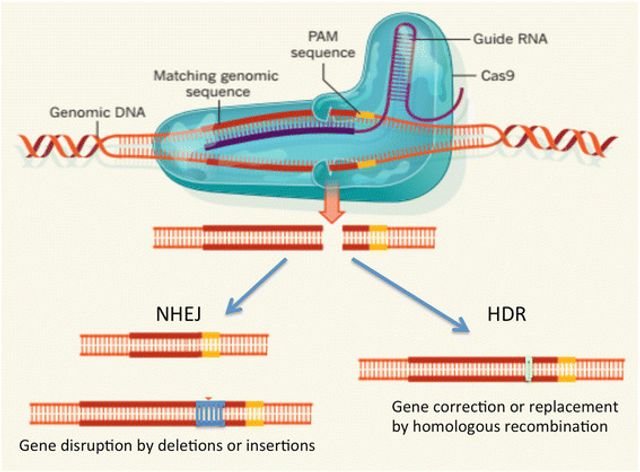 The CRISPR-Cas9 editing tool uses an RNA guide to fix mutated parts of DNA by breaking the DNA strands, excising the undesired genetic sequence and then rebuilding the molecule
The CRISPR-Cas9 editing tool uses an RNA guide to fix mutated parts of DNA by breaking the DNA strands, excising the undesired genetic sequence and then rebuilding the molecule
The authors of the study explain that CRISPR-Cas9 was being used to introduce mutations in genes and observing the impact on the development of the embryos. Because the HDR method is inefficient, gene editing for gene therapy had been thus far limited. Additionally, earlier attempts at corrective gene editing in one-cell embryos led to creating a mosaic offspring carrying the corrected as well as the defective cell type. Theoretically, the scientists were concerned that in attempting to correct a genetic defect new mutations would be introduced.
In an attempt to correct these problems, the researchers injected a sperm carrying the MYBPC3 defect into an oocyte (female egg) obtained from a healthy donor using the CRISPR-Cas9 technique. What they found was that introducing CRISPR-Cas9 at an earlier stage led to more efficient excision and a preference for the developing embryo to use the normal DNA from the mother to correct broken DNA—the HDR method for correction. This was a considerable surprise for the investigators, leading to a hypothesis that “human embryos employ different DNA repair mechanisms than do somatic or pluripotent cells, probably reflecting evolutionary requirements for stringent control over genome fidelity in the germ line.”
Using CRISPR-Cas9 in this manner produced embryos in which 82.4 percent had normal genes from both the mother and father. Moreover, as these embryos were allowed to divide into blastocysts, repeat genomic analysis demonstrated that the healthy DNA strands successfully replicated themselves in most cases.
 Illustration of injecting an ovum (egg) with a donor sperm and the CRISPR-Cas9 enzyme
Illustration of injecting an ovum (egg) with a donor sperm and the CRISPR-Cas9 enzyme
More work is required to ensure sufficient safety standards before this technology is ready for clinical use. There may be other ways of increasing the percentage of healthy embryos and reducing the chance that the corrected DNA mutates again. Teams will need to correct genetic disorders other than HCM. More broadly, the gene editing technique will need to be further tested to ensure that other genetic disorders aren’t accidentally introduced. Ultimately, the process will need to be tested during an actual pregnancy.
As for the fears cited by some, Alta Charo, a bioethicist at the University of Wisconsin at Madison, stated that she doubts a flood of couples will have “edited” children. She told the New York Times, “Nobody’s going to do this for trivial reasons. Sex is cheaper, and it’s more fun than IVF, so unless you’ve got a real need, you’re not going to use it.”
There are of course class questions regarding the creation of “designer babies.” The current experiments are privately funded, as reactionary laws prohibit US tax dollars from being used in embryo research. This means that the process of curing genetic disorders could become patented and turned into a source of profit, only available to the select few who are able to afford it. Or it could be weaponized by military contractors like Academi (formerly Blackwater and Xe Services) in order to make genetically engineered soldiers hired out to kill at the behest of US imperialism.
Such questions highlight the contradiction of the developments in genetic engineering. Their potential for abuse is high. At the same time, the fact that humanity has the scientific and technical prowess necessary to correct a defect in the DNA of a human embryo is a significant advance in our understanding of the development of life.